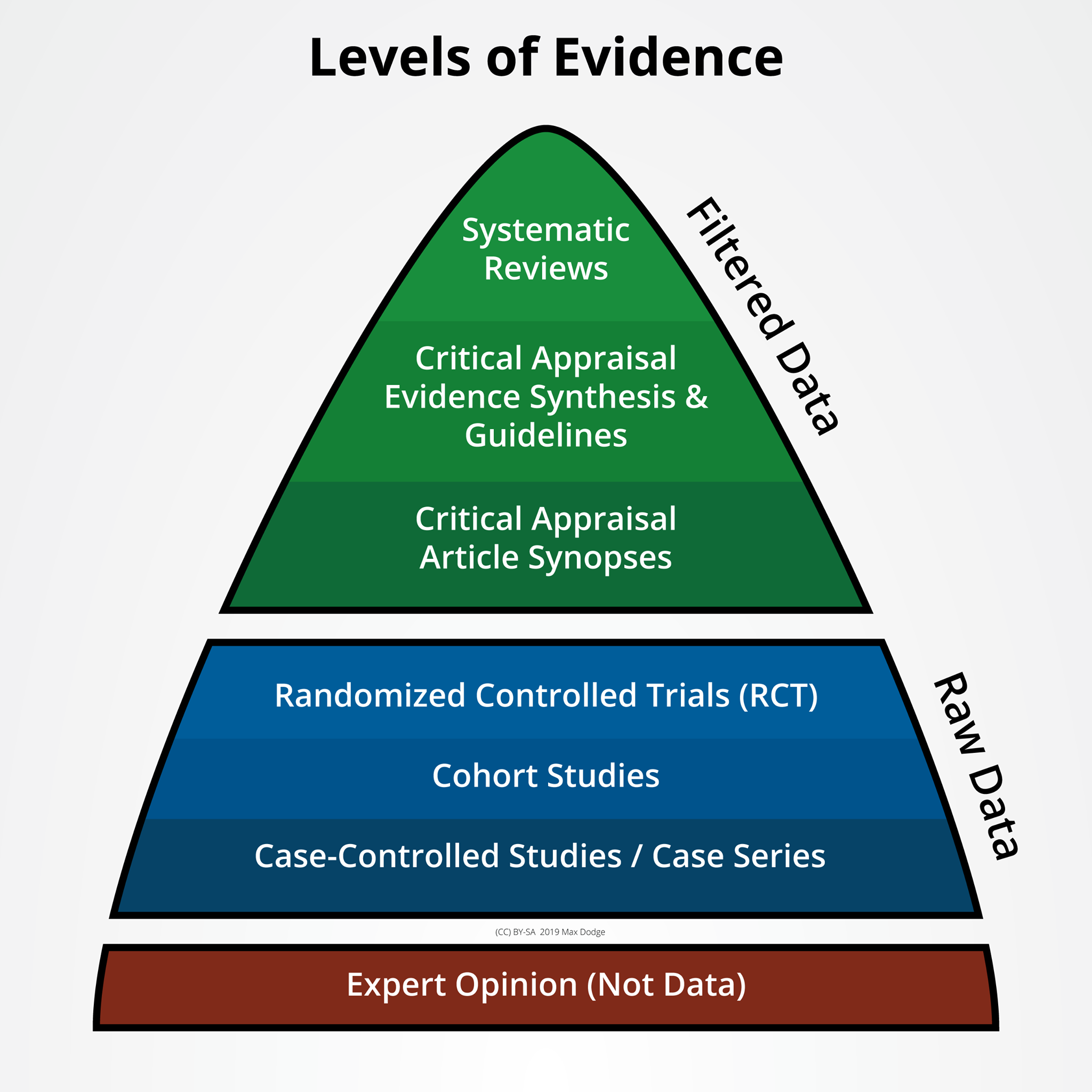
Levels of evidence (sometimes called hierarchy of evidence) describe studies based on the methodological quality of their design, validity, and applicability or generalizability to larger patient populations.
The highest levels of evidence involve the filtering and consolidation of information. These products are usually the most reliable in their conclusions or recommendations. This includes systematic reviews or meta-analyses of all relevant RCTs (randomized controlled trial) or evidence-based clinical practice guidelines based on systematic reviews of RCTs or three or more RCTs of good quality that have similar results.
The next group of evidence levels involves raw data from well-constructed studies. A well-designed study is constructed to remove potential bias and powered to show a statistically and clinically significant result. Collectively, these may be reviewed for meta-analysis or systematic review.
The lowest form of evidence does not involve data. However, evidence from the opinions of authorities and/or reports of expert committees may still provide important insight into problems.